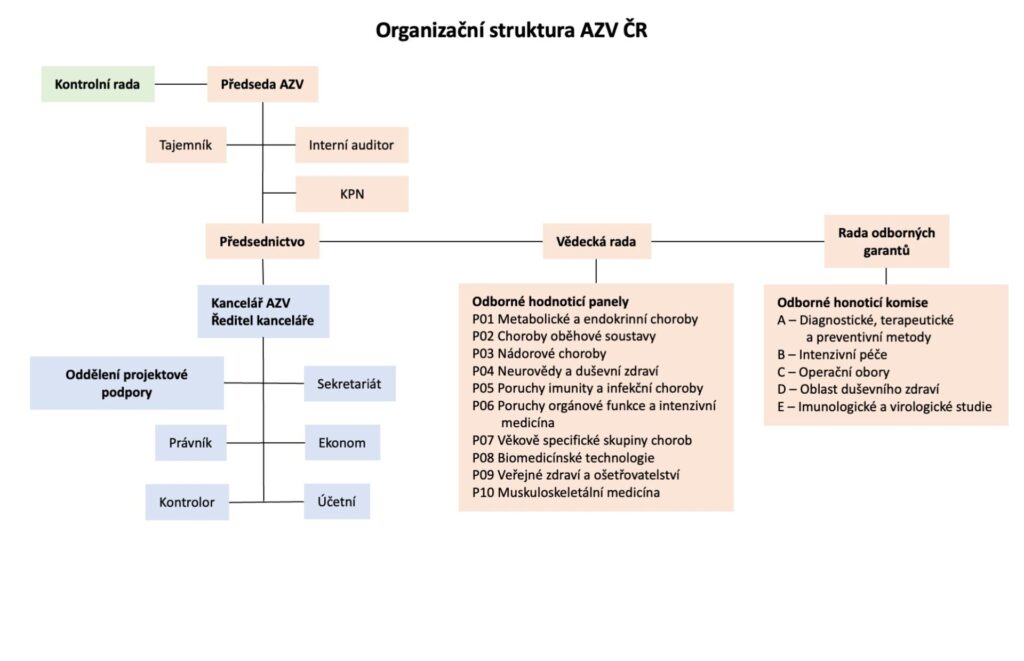
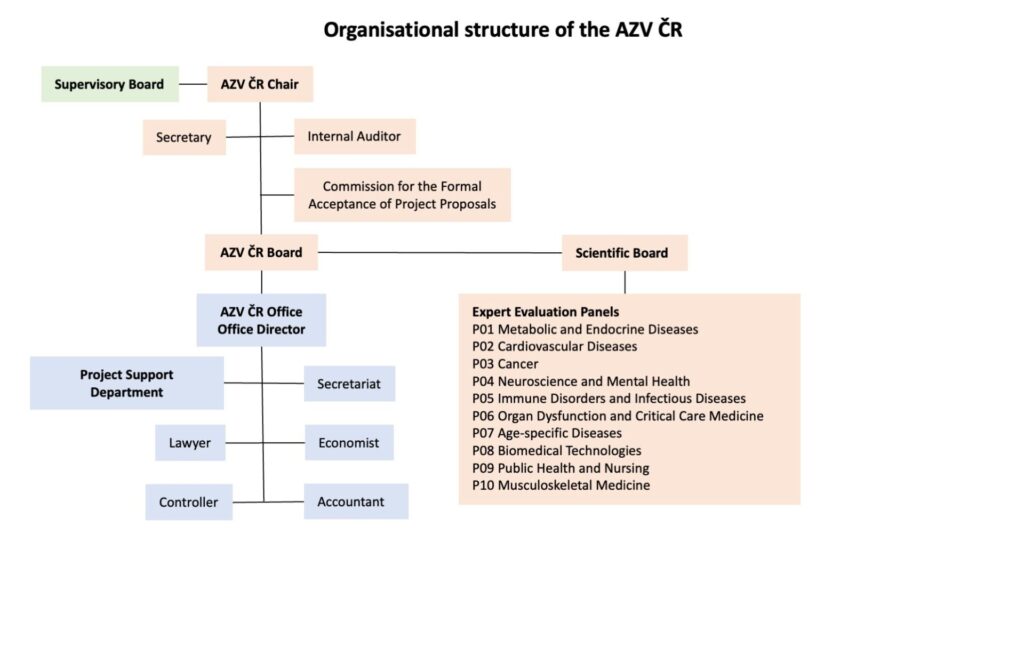
Agency for Health Research of the Czech Republic (hereinafter referred to as "AZV") is an organisational unit of the State under the direct management of the Ministry of Health of the Czech Republic, with its registered office at Ruská 85, 100 05 Praha 10, ID No. 03009491. It is an independent accounting unit.
AZV was established by the Measure of the Ministry of Health by the Charter of the Ministry of Health dated 1 April 2014 under No.: MZDR 10256/2014/VLP as amended by the Measure of the Ministry of Health (hereinafter referred to as "MH") issued under No.: MZDR 51831/2016-1/OPŘ dated 6 September 2016.
The basic purpose of AZV is to support applied research in the health sector in accordance with Act No. 130/2002 Coll., on the Support of Research, Experimental Development and Innovation from Public Funds and on Amendments to Certain Related Acts (Act on Support of Research, Experimental Development and Innovation), as amended, hereinafter referred to as "the Act".
AZV within the scope of its activities, it ensures in particular:
a) Preparation of programmes and other activities in the field of applied biomedical research, including public competitions in research, development and innovation in support of programme projects (hereinafter referred to as "the Project");
b) evaluation and the selection of Project proposals for the provision of targeted support for their solution;
c) Preparation of documents for the provision of earmarked support to Projects on the basis of support contracts or decisions on budget increases;
d) Professional and substantive control of selected supported Projects and cooperation with the Ministry of Health in controlling the implementation of contracts for the provision of support or decisions to increase the budget and the use of earmarked support;
e) evaluation and control of the progress of the solution and the fulfilment of the objectives of the Projects and control of the results achieved by them;
f) Preparation of expenditure proposals AZV and reports on its activities;
g) Controlling the fulfilment of the Project objectives within the meaning of §13 (2) and (3) of the Act;
h) Activities resulting from approved European Projects where AZV is a beneficiary of subsidies;
i) Negotiations with the competent authorities of the Czech Republic or the European Union on the assessment of the compatibility of the aid granted with the common market;
j) Cooperation with similar foreign agencies;
k) Archiving of documents during the course of the Projects, including the period of 180 days from the date of completion of the Project necessary for the evaluation of the Project. Thereafter, all documents will be handed over to the MoH for archiving in accordance with Act No. 499/2004 Coll., on Archives and Records Management and on Amendments to Certain Acts, as amended;
l) Performing other tasks on the basis of the mandate of the MZ.
AZV provides documents for the provision of special-purpose support in accordance with the National Policy of Research, Experimental Development and Innovation of the Czech Republic, on the basis of a public competition in research, development and innovation under the Act, for Programme Projects in which the recipient expresses how and under what conditions it will contribute to the fulfilment of the objectives of the Programmes (Section 3(2)(b) of the Act).
The earmarked support is provided from the expenditure of the Ministry of Health in the form of a subsidy to legal or natural persons or by increasing the expenditure of organisational units of the Czech Republic or local self-government units (Section 4(1) of the Act).